Designated Marketing Authorization Holder (DMAH) Service
Proposal for Designated Marketing Authorization Holder (DMAH) Service for Corporations
Designated Marketing Authorization Holder (DMAH) Service
When a foreign manufacturer sells a product in Japan, even if it does not have a place of business in Japan, it can become an applicant for approval and hold a Japanese approval on its own. In such cases, a domestic marketing authorization holder must be appointed as the marketing authorization holder (Designated Marketing Authorization Holder/DMAH).
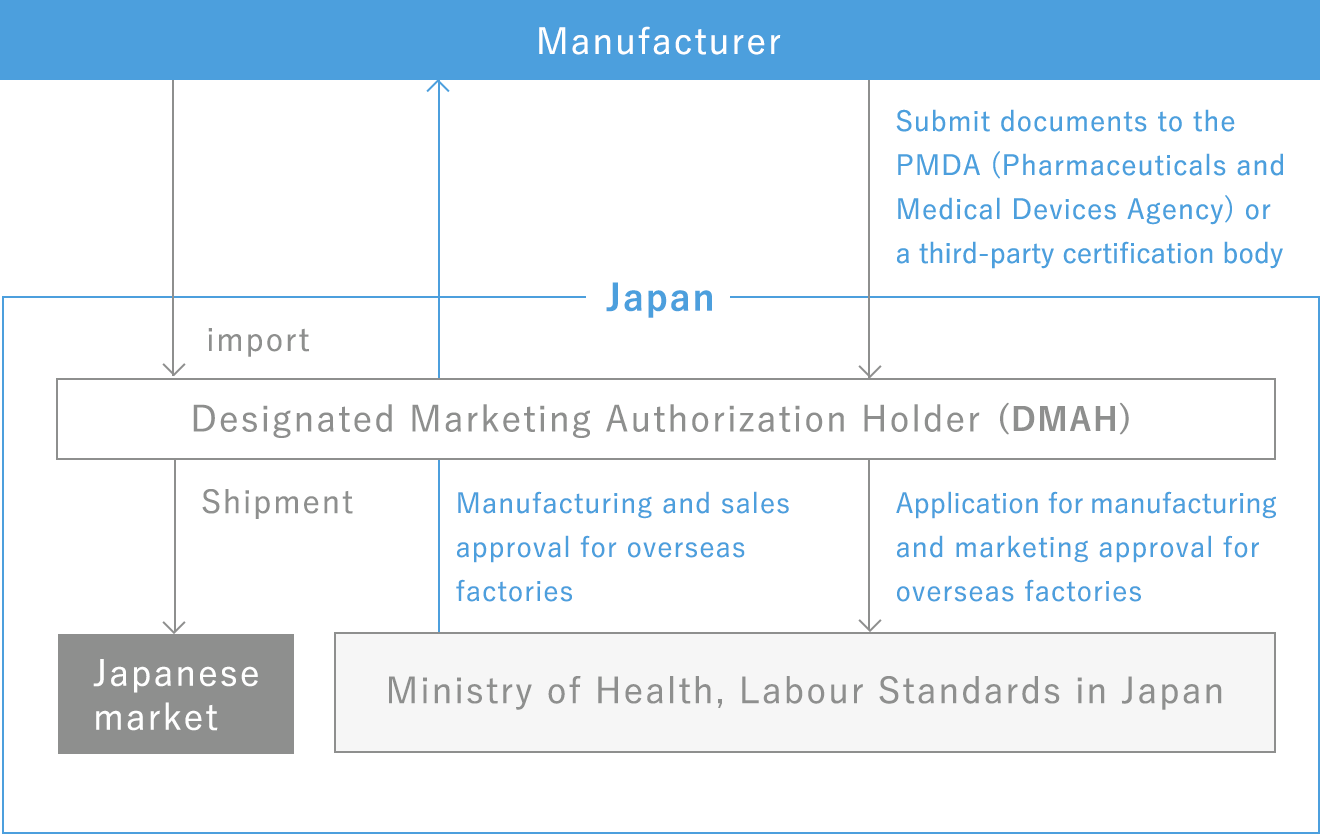
The DMAH is obligated to conduct manufacturing and quality control in Japan in accordance with the QMS Ordinance, safety control operations in accordance with the GVP Ordinance, and implementation and coping of operations related to the product.
By appointing a DMAH, you can select a distributor and sales network, allowing you to control your own distribution.
We have obtained First-class medical device manufacturing and Sales license, Medical device manufacturing license, and Specially-controlled medical device sales license, and import and sales of medical devices as a manufacturer and distributor, and are familiar with the methods from obtaining approval to import and sales. We have a track record of outsourcing pharmaceutical affairs consulting services from overseas manufacturing sites.
We will DMAH support
-
Regulatory affairs strategy planning, investigation of application categories for approval, PMDA consultation,
and application submission on behalf of the client. -
Preparation of application forms for foreign manufacturer registration and application on behalf of the applicant.
-
Preparation of application documents for Medical Device and inquiries correspondence from a review authority.
-
Application procedures for changes or updates to product design, manufacturing sites, etc.
-
Support for QMS conformity surveys related to quality.
-
Quality control operations for imported products.
-
Shipping to the distributor.
-
Customs clearance and other related work when importing medical devices.
-
Safety management services for medical devices.
-
Response to product failures and adverse events in Japan.
Frequently Asked Questions (FAQ)
- Q1 Is it possible to only apply for foreign manufacturer accreditation or registration?
- A We can provide various services such as preparation of application documents and procedures for approval, adverse of manufacturing plants. Our professional staff will respond to your requests.
- Q2 Is it possible to have a partial change in an approved item or an application for a minor change?
- A Our staff, who are familiar with the approval of medical devices, can respond quickly.
- Q3 I would like to know about the quality control and safety management operations of products after they are put on the market.
- A We have obtained First-class medical device manufacturing and Sales license, Medical device manufacturing license, and Specially-controlled medical device sales license. We can provide support according to your needs, from product quality control and product import procedures to shipping to market.
- Q4 I would like to you to respond to QMS conformity surveys at manufacturing plants and take corrective actions accordingly.
- A We import medical devices from overseas manufacturing plants and sell them globally. Our experienced staff can provide support for QMS and GVP compliance at manufacturing plants.